Back in the early days of organic chemistry, chemists knew that molecules were collections of atoms held together by chemical bonds. However, the exact 3D shapes of these molecules were a mystery since they couldn’t be directly observed. Chemists often used flat diagrams to show how atoms were connected, but these were not sufficient to explain many of their observations. For a long time, the chemical theory couldn’t provide a satisfactory explanation for the three-dimensional structures.
In 1874, a break came when Van’t Hoff proposed that the four bonds of a saturated carbon atom point to the corners of a tetrahedron. This was a game-changing idea, although it took another 25 years for the quantum theory to back it up. Van’t Hoff used optical rotation to support his hypothesis, noticing that only compounds with a central carbon bonded to four different atoms or groups could rotate plane-polarized light.
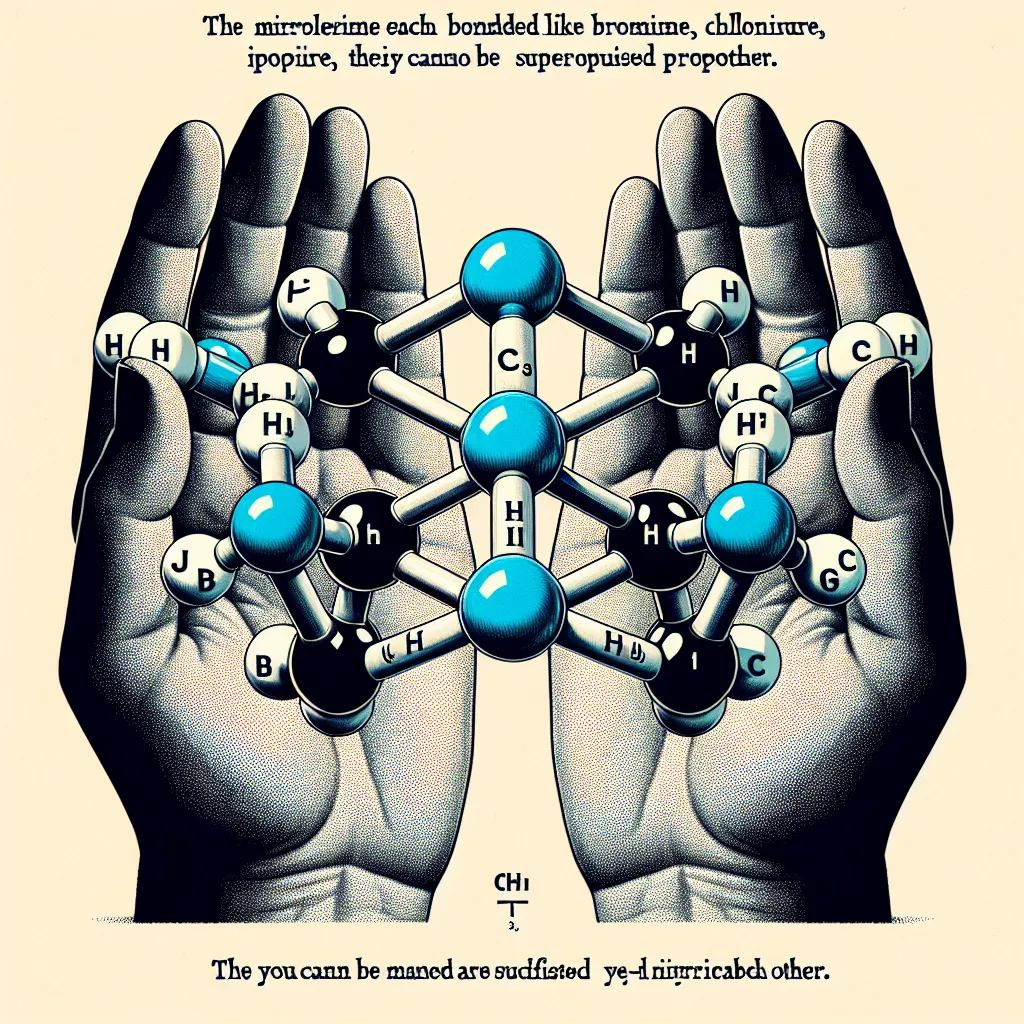
Imagine two molecules each with a central, tetrahedral carbon atom bonded to bromine, chlorine, fluorine, and hydrogen. At first, you might think these molecules are identical, but no matter how you rotate or translate them, you can’t perfectly superimpose one on the other.
Think about your hands. They have the same parts: thumb, fingers, palm. They look similar and have the same layout, yet they are not identical. Try overlapping them—it’s impossible to do perfectly. When you point your palms at each other and wiggle your index fingers, one hand looks like a mirror image of the other. This mirror-image property is also seen in our molecules. This property is called chirality or handedness.
A chiral object is not the same as its mirror image. Chirality is significant in both chemistry and daily life. For example, screws are chiral, which is why we have right-handed and left-handed screws. Certain types of light also behave like chiral screws, rotating in a way that produces plane polarization. When chiral molecules are placed in this light, they interact differently with the right-handed and left-handed components, causing optical rotation.
Van’t Hoff and other chemists later discovered that chirality in tetrahedral carbons could explain this fascinating optical rotation. Chirality is responsible for many interesting effects in both chemistry and daily life. While humans tend to create symmetrical objects, chiral molecules are ubiquitous in nature. Whether it’s optical rotation, assembling furniture, or clapping hands, chirality is a fundamental and intriguing property.
So, understanding chirality and the 3D structure of molecules has been crucial in chemistry. It sheds light on why certain compounds behave the way they do, impacting everything from the study of light to everyday tasks.