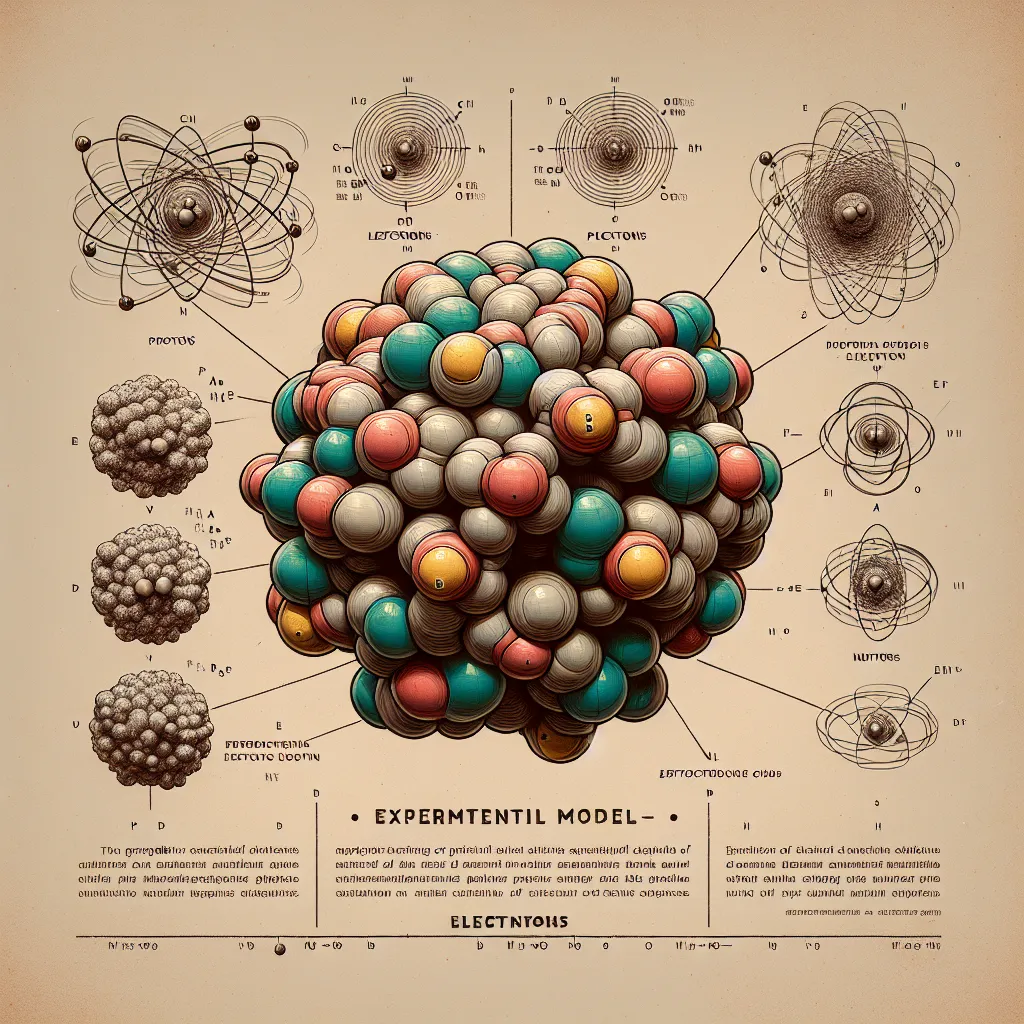
You might remember from science class that everything is made up of atoms. Atoms are super tiny particles composed of a core with positively charged protons and, usually, neutral neutrons. Surrounding this core are negatively charged electrons. What makes each atom unique? It’s the number of protons in its nucleus. For example, hydrogen has one proton, carbon has six, and gold has 79.
Let’s take a quick detour. How do we even know what atoms look like if we can’t see them? It’s all about experiments. Scientists conduct tests, come up with models, see if the results fit the model, and then repeat the process. If things don’t match up, a new model is proposed. This has been happening since ancient times, starting with Democritus in 400 BC, and it’s likely to continue.
The core of an atom stays together, but electrons move around freely, which is why chemists are obsessed with them. Electrons are peculiar because they can act like particles or waves, depending on how we examine them. One of the strangest things is that we can’t precisely locate an electron. It’s not about technological limits; it’s just how electrons behave. Instead, we talk about the probability of finding an electron in certain regions around the nucleus.
So, if we were to draw a shape around the nucleus where we’re 95% sure to find an electron, what would it look like? These shapes are called orbitals, and they vary based on the electron’s energy. The higher the energy, the farther its density is from the nucleus. But why 95% and not 100% certainty? Beyond a certain distance, the likelihood of finding an electron drops exponentially, never quite reaching zero. This means that technically, there’s always a tiny chance an electron could be on the other side of the universe, but mostly they stay close, forming these dense clouds around the nucleus.
How electrons from different atoms interact shapes almost everything. Atoms can give away, take, or share electrons, and this interaction is what makes chemistry so fascinating. From simple rocks to the intricate fabric of life, the atomic level defines everything we experience through our senses.